|
Effect of Acyclovir on Rat Fetus Palate Mucosa
Marilena Chinali KOMESU
Luiz Guilherme BRENTEGANI
Reinaldo AZOUBEL
Ruberval Armando LOPES
Miguel Angel SALA
Departamento de Estomatologia, Faculdade de Odontologia de Ribeirão
Preto, Universidade de São Paulo, Ribeirão Preto, SP, Brasil
Braz Dent J (1995) 6(1): 17-23 ISSN 0103-6440
| Introduction | Material/Methods
| Results | Discussion
| Conclusions | References
|
Five pregnant rats were treated during organogenesis with sc injections
of acyclovir (50 mg/kg body weight) on days 9, 10 and 11 of pregnancy.
The fetuses (N = 62) were evaluated on day 20 of gestation and presented
decreased body weight as well as delayed differentiation of fetal rat palate
epithelium, with increased nuclear volume, decreased cytoplasmic and cellular
volumes, decreased epithelial and keratin thicknesses, and increased cellular
numerical density.
Key words: Acyclovir, palate, rat fetus, stereology.
Introduction
Acyclovir has been shown to have teratogenic properties in rats and chicks
producing gross malformations which vary according to the dose and the
embryonic age at the time of injection (Stahlmann, 1988). Administration
of acyclovir to rats during organogenesis resulted in anomalies of the
skull (reduction of the crown-rump length, abnormal shape of the head,
decreased width of the skull, resembling a beak-like visceral cranium,
smaller or missing os timpanicum), anomalies of the vertebral column, missing
tail, and protruding tongue (Stahlmann et al., 1988; Chahoud et al., 1988).
The present study was undertaken to determine the alterations present in
palatine mucosa induced by maternal injection of acyclovir, during the
teratogenic period in rats.
Material and Methods
Wistar rats (N = 10) were kept under constant day/night cycle (12/12 h),
relative humidity and at room temperature (23 + 2oC). They were fed Purina
pellet feed and tap water ad libitum. One male was caged with two females
overnight and females were examined the following morning for the presence
of sperm in the vaginal smear. The day sperm were detected was designated
as day one of gestation.
The commercially available preparation for intravenous application of
acyclovir (sodium salt) was used (ZoviraxÒ; Wellcome Chemical Works,
Dartford, Kent, England). Five animals were treated subcutaneously on days
9, 10 and 11 of gestation with 50 mg acyclovir/kg body weight. Control
animals were treated similarly with saline.
The animals were sacrificed on day 20 of gestation, and the fetuses
were removed. To examine for variations in pathology within and among litters,
five complete litters of each group were fixed in a solution of 85 ml 80%
ethanol, 10 ml formol, and 5 ml glacial acetic acid, and after processing,
embedded in paraffin and serially sectioned at 6 mm. The sections were
stained with hematoxylin and eosin.
Stereological analysis was performed with the curvilinear test system
of Merz (1968). The following parameters were calculated after point counting
(720 per animal) on palatine epithelium: nuclear, cytoplasmic and cellular
volumes, nuclear/cytoplasm ratio, keratinized surface density, epithelial
thickness, keratin thickness, free surface/basal surface ratio, and numerical
density (described in detail by Ribeiro et al., 1990).
Comparison of the results for the experimental group and the controls
was performed by the non-parametric Mann-Whitney test.
Results
The mean fetal body weight was 5.07 g for the control group and 3.83 for
the acyclovir-treated group (P<0.05).
Histologically, the hard palate epithelium of experimental animals was
thinner and less differentiated than in control fetuses. The epithelium
was composed of smaller and more numerous cells, with larger nuclei. The
keratin layer was thinner. The alterations in the soft palate epithelium
were less evident. The mean values of stereological parameters of the epithelial
cells from both hard and soft palates can be observed in Table 1.
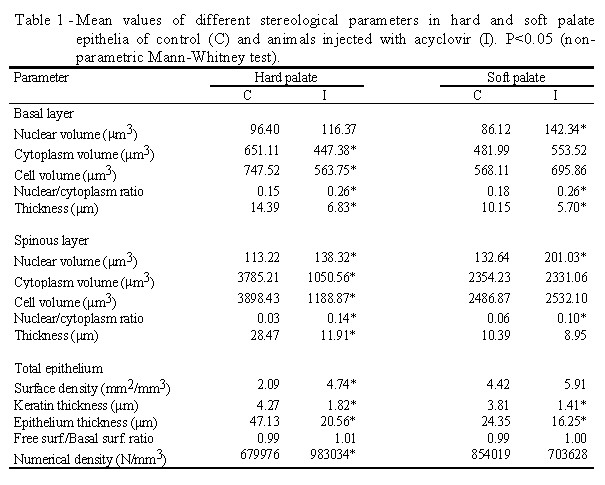
The nuclear volume of the basal and spinous cells from either hard or
soft palates was larger in the treated group. Statistical analysis showed
a significant difference between the control and the treated fetuses only
in the soft palate for basal cells, and significant in both palates for
spinous cells. The cytoplasmic and cellular volumes for basal and spinous
cells from hard palate were significantly smaller in the treated group.
The mean values of the cytoplasmic and cellular volumes in cells from hard
and soft palates were significantly smaller in treated fetuses.
Mean epithelial surface density was smaller in the control group than
in the treated group. Statistical analysis showed the existence of a significant
difference between the groups only in the hard palate. The hard and soft
palate epithelia were significantly thinner in the treated fetuses than
in the control ones. Keratin thickness was also decreased.
Table 1 shows that the values for the free surface/basal surface ratio
were no different between groups. The number of cells by cubic millimeter
of epithelium of hard palate was greater (P<0.05) in treated animals
than in the controls, whereas in the soft palate the values were similar.
Discussion
Epithelial alterations were observed in hard and soft palates of fetuses
whose mothers were injected with 50 mg acyclovir/kg body weight on days
9, 10 and 11 of pregnancy. These alterations were shown by stereology (larger
basal and spinous nuclei and smaller cell volumes, thinner epithelial and
keratin thicknesses, and greater numerical density). These findings demonstrate
a delayed differentiation of the analyzed tissue in treated animals.
Acyclovir [9-(2-hydroxyethoxymethyl) guanine] is used as a specific
virustatic agent for the treatment of infections with herpes-type viruses.
With the aid of a specific virus-coded kinase, acyclovir is phosphorylated
to the corresponding triphosphate (Furmann et al., 1981) and incorporated
into the viral genome during replication. Inhibition of DNA synthesis (Furmann
et al., 1979) by termination of DNA chain growth is assumed to be responsible
for the virocidal effect observed.
All current drugs which inhibit viral DNA synthesis also affect the
DNA of uninfected cells. Acyclovir shows an unusually high degree of selectivity:
its conversion to the monophosphate is catalyzed with a high degree of
specificity by the viral thymidine kinase. However, it is well known that
at high concentrations an effect on the DNA metabolism of uninfected cells
also occurs (Stahlmann et al., 1988). These findings explain the alterations
observed in the epithelium in this study.
The dose of 3 x 50 mg acyclovir/kg daily, used in this research, exhibited
no teratogenic effect. Acyclovir exhibited no teratogenic effect when administered
to rats at a sc dose of 3 x 25 mg acyclovir/kg daily from day 6 to 15 of
gestation (Moore et al., 1983). Eight doses of 50 mg acyclovir/kg during
organogenesis (from early day 9 to day 11) induced no defects, but a single
injection of 200 mg acyclovir/kg on day 10 produced severe head defects
(Stahlmann et al., 1988). Information on the teratogenic potential of acyclovir
without maternal influence comes from the rat whole-embryo in vitro studies
(Klug et al., 1985).
The reduced fetal weight observed in this study was also present in
the findings of Chahoud et al. (1988). Acyclovir reduces the placental
weight in rats (Komesu et al., 1995), and small placentas have impaired
circulation with reduced blood flow to the fetus and thereby impair nutrition,
which results in fetuses with smaller body weight.
Conclusions
Prenatal exposure to acyclovir in rats results in developmental alterations
characterized by a decrease in body weight as well as by the delayed differentiation
of diverse organs and tissues, including the epithelium of hard and soft
palates.
Acknowledgments
Research supported by CNPq (Proc. 300.535/90-2).
References
Chahoud I, Stahlmann R, Bochert G, Dillmann I, Neubert D: Gross-structural
defects in rats after acyclovir application on day 10 of gestation. Arch
Toxicol 62: 8-14, 1988
Furmann PA, St Clair MH, Fyfe JA, Rideout JL, Keller PM, Elion GB: Inhibition
of Herpes simplex virus-induced DNA polymerase activity and viral DNA replication
by 9-(2-hydroxyethoxymethyl) guanine and its triphosphate. J Virol 32:
72-77, 1979
Furmann PA, de Miranda P, St Clair MH, Elion GB: Metabolism of acyclovir
in virus-infected and uninfected cells. Antimicrob Agents Chemother 20:
518-524, 1981
Klug S, Lewandowski C, Blankenburg G, Merker HJ, Neubert D: Effect of
acyclovir on mammalian embryonic development in culture. Arch Toxicol 58:
89-96, 1985
Komesu MC, Brentegani LG, Lopes RA, Sala MA, Azoubel R, Garcia Lima
E: Morfologia de fetos de ratas injetadas com aciclovir no 10o dia da gestação.
Rev Reg Ciências 4: 151-156, 1995
Merz WA: Die Streckenmessung an gerichteten Strukturen im Mikroskop
und ihre Anwendung zur Bestimmung von Oberflächen-Volumen-Relationen
im Knochengewebe. Mikroskopie 22: 132-142, 1968
Moore Jr HJ, Szczech GM, Rodwell DE, Kapp Jr RW, de Miranda P, Tucker
Jr WF: Preclinical toxicology studies with acyclovir: teratologic, reproductive
and neonatal tests. Fund Appl Toxicol 3: 560-568, 1983
Ribeiro CAL, Maia Campos G, Melis MS, Sala MA, Lopes RA: Alteraciones
morfológicas del epitelio lingual de fetos de rata provocadas por
el ejercício materno. Arch Fac Med Zaragoza 30: 84-88, 1990
Stahlmann R, Klug S, Lewandowski C, Bochert G, Chahoud I, Rahm U, Merker
H-J, Neubert D: Prenatal toxicity of acyclovir in rats. Arch Toxicol 61:
468-479, 1988
Correspondence:Prof. Dr. Ruberval A. Lopes, Departamento de Estomatologia
(Patologia), Faculdade de Odontologia de Ribeirão Preto, USP, 14040-904,
Ribeirão Preto, SP, Brasil.
|

